Whenever a new testing mechanism for Coronavirus testing is approved by the FDA, within days dozens of competing options are released into the marketplace.
One of the most popular rapid tests for SARS-CoV-2 that has gained international interest are rapid antigen tests.
Unlike a rapid antibody or serology test which detects whether the patient’s immune system has reacted to the presence of SARS-CoV-2, a rapid antigen test for COVID-19 is somewhat like a qPCR test in that it is searching for the presence of Coronavirus in the patient.
While qPCR detects genetic material, a rapid antigen test searches for the tell-tale spike protein which is an important part of the virus and unique to each coronavirus.
By far the most affordable and available rapid antigen tests are lateral flow tests.
Using a simple biochemical process and inexpensive materials, a lateral flow rapid antigen test is highly accurate for diagnosing a symptomatic patient with COVID-19 within 5-7 days of developing symptoms; somewhere between 88-97% of the time depending on the rapid antigen test.
When choosing a rapid antigen test for Coronavirus, there are a few things that distinguish one test kit from another.
At a minimum, all lateral flow rapid antigen tests will contain plastic test cassettes (twenty seems to be the industry standard), an equal number of tube/droppers for mixing and depositing the sample into the cassette and a dropper of buffer which contains a substance to break open the cellular material in the sample so the spike protein can be freed and then detected by the test (called a cell-lysis buffer in scientific terms).

Because the lateral flow rapid antigen test cassettes contain a chemical compound, they should be individually wrapped in a light-safe aluminum pouch for sterility and to maintain potency.
Depending on the manufacturer and the different types of samples effective with that test, the box may include nasal or oral swabs for collecting samples and sterile collection cups if sputum can be used.
Rapid antigen tests DO NOT require a finger-prick of blood. These are not rapid antibody tests.
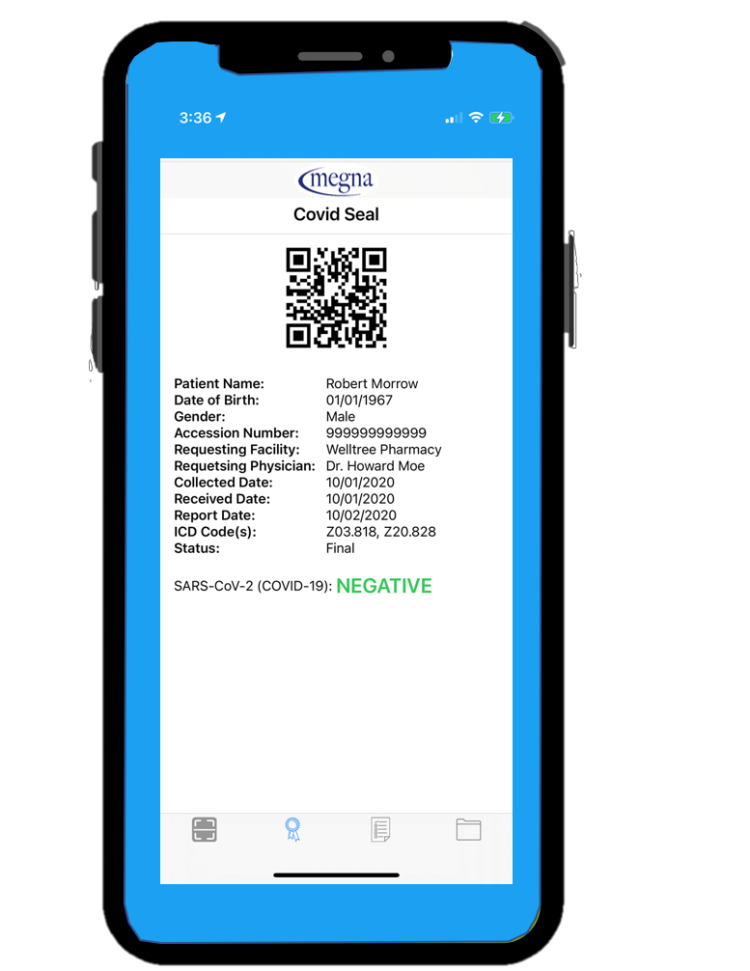
Some manufacturers of rapid antigen tests, like this pending EUA one from Megna Health, imprint a QR code on their cassettes which can be read by a free app made available for download.
While not technically “in the box” this nice feature makes it easy for healthcare providers to quickly log test results and include them in their patient records.
Different lateral flow rapid antigen tests deliver results in a varying range of time. Whether it is 10-15 minutes or closer to 30 minutes, the rapid antigen test must be read during this time when the chemical reaction is most prominent.
Because this can allow room for human error, it is important to have properly trained staff on hand to monitor the COVID-19 test results while other tests are being set up and conducted.
Stellar Scientific offers several choices of rapid antigen tests for Coronavirus including the popular Access Bio Carefirst rapid antigen tests that carry an FDA EUA (emergency use authorization) and are CLIA waived so they may be performed by a wide range of medical offices and practices.


