While it is nice to fantasize that the arrival of the Pfizer and Moderns mRNA vaccines for SARS-CoV-2 will make testing a thing of the past, until some degree of herd immunity is achieved, testing for Coronavirus will continue, and probably for many years to come.
While PCR testing continues to be upheld as the “gold standard” for COVID-19 testing, it is a complex testing process that is dependent on increasingly hard to find supplies which can stretch out TAT (turnaround time) and hamper efforts to contain the spread of the virus.
Recently a new form of testing that promises faster results has become available, a rapid antigen test for Coronavirus.
Rapid antigen tests have diagnostic value as they look for the presence of the SARS-CoV-2 virus and can identify a person with an active infection.
While a RT-PCR test is designed to look for the genetic material contained in the COVID-19 virus, a rapid antigen test looks for a specific antigen protein that is a telltale sign of the virus.
In the case of Coronavirus, rapid antigen tests are designed to identify the “spike protein” which gives the Coronavirus its unique look and identity.
As with all COVID-19 tests, rapid antigen tests are graded for their “sensitivity” and “specificity.”
Sensitivity says how well the test detects its target while specificity says how confidently we can say the target was correct and not something else.
There are several different kinds of rapid antigen tests now on the market.
Device based rapid antigen tests like the Quidel Sophia 2 and the BD Veritor Plus use a sophisticated instrument to look for the spike protein antigen.

Device based rapid antigen tests offer a high level of confidence in accuracy because the machine reads the results and are not prone to human error.
However, devices like the Quidel Sophia 2 and BD Veritor require costly capital outlay to purchase the unit as well as specific consumables needed to operate the rapid antigen test correctly.
Stellar Scientific offers inexpensive and highly accurate lateral-flow diagnostic rapid antigen tests from a few different manufacturers.
A lateral flow test uses a very simple biochemistry principle to produce reliable and affordable rapid antigen tests.
A piece of absorbent nitrocellulose paper is impregnated with a colorimetric target that changes colors when it detects and reacts with the spike protein.
The paper is encapsulated in a plastic cassette with simple to follow instructions; where to deposit the sample and where to add the buffer (in some cases) that causes the sample to migrate towards the target.
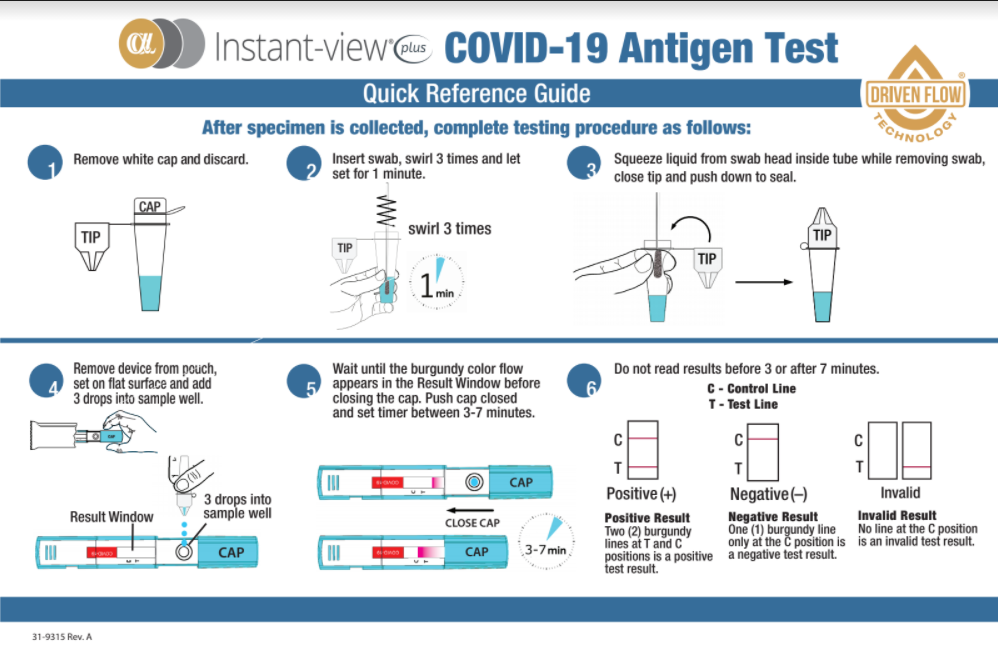
Patient samples for a rapid antigen test are typically collected in the same manner as samples for a qPCR test, using either a nasal or oral swab although there are some tests, like the Megna Health rapid antigen test, which can use sputum as well.
The spike protein on the SARS-CoV-2 virus is what triggers an antibody and immune system response, so there is a limited window of time when these tests can be performed and assumed to be highly accurate before the body begins destroying the protein in self-defense.
The current recommendations are to use a rapid antigen test to screen symptomatic patients within the first 5-7 days of showing symptoms.
During this time there may be sufficient spike protein in the sample to be detected as the infection is at its most potent phase.
Rapid antigen tests are not recommended for testing asymptomatic patients.
During the symptomatic time frame some rapid antigen tests claim a sensitivity of nearly 97% making them highly accurate.
Rapid antigen tests that have an EUA from the FDA can be CLIA waived which allows them to be purchased by a wide range of medical professionals and not just highly trained clinical laboratories.


